2016-06-17 15:43
Drug registration system will be built to meet the reform of drug listing permit holders
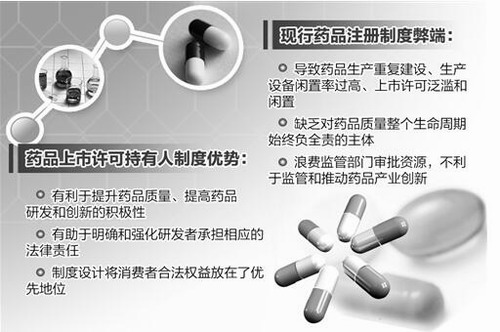
[straight news network Beijing June 17 news] (China Economic Net) the general office of the State Council recently issued the listed drugs license holder system pilot scheme ", in Beijing, Tianjin, Hebei, Shanghai, Jiangsu, Zhejiang, Fujian, Shandong, Guangdong, Sichuan the province (city) to carry out drug listed pilot license holder system, allows the medicine research and development institutions and researchers drug approval numbers, assume corresponding responsibility for the quality of medicines.
"The establishment of the drug market permit holder system is the core content of the drug registration system reform in China." Tsinghua University School of law, health law research center, pharmacy law research institute director Wang Chenguang said that the pilot is not only beneficial to the new drug research and development institutions and researchers actively created to encourage pharmaceutical innovation, to enhance the quality of drugs also has an important significance. In drugs listed license holder system pilot as a breakthrough, China's drug registration system will by "bundling" system of the marketing license and production license, to the public license and production license from the marketing authorization holder system "transformation.
The drawbacks of the current drug registration system
Drugs are a special commodity which is related to people's life and health. People looking forward to the use of drugs at the same time, but also look forward to more good medicine to enter the market.
"The current drug registration system is listed bundling license and production license management mode, namely only pharmaceutical producing enterprises can apply for drug registration, drug approval numbers." State Food and Drug Administration of the legal department director Xu Jinghe said, in the market order has not yet been established, social innovation ability is limited, enterprises mainly to produce generic drugs, with the production of pharmaceuticals as the basis for registration and regulation still has certain rationality. However, with the gradual improvement of the market economic system of our country, to continue to develop the ability of research and development of pharmaceutical industry innovation, masses of safe and effective drug demand growing, this "bundling" registration management is increasingly obvious shortcomings has become an important factor restricting the further development of our country medicine conduct industry.
It is understood, some production enterprise for the pursuit of market efficiency, expanding pharmaceutical production of varieties or construction of new production lines, resulting in pharmaceutical production and repeated construction and production equipment idle because of the high rate of false prosperity. A few more enterprises to formulations, packaging, specifications different grounds duplicate reporting, approval number, cause marketing authorisation flooding and idle, the impact of China's pharmaceutical industry healthy and orderly development and innovation.
In addition, the current permission system did not clearly defined drug producers, operators and medical institutions related to the subject of liability, resulting in the pipe section, not the whole life cycle of drug quality always negative full responsibility for the subject, even if the patients' rights to effective legal guarantee, and can not guarantee the quality of medicines throughout the life cycle of the monitoring system.
Wang Chenguang, said "tied a lack of intrinsic system fully proves that the system and China's pharmaceutical industry development does not adapt, leads not only to regulatory authorities to waste a lot of resources at a low level repeated declaration review and approval, to form the effective supervision of the whole life cycle of a drug, unable to promote the pharmaceutical industry innovation, it is difficult to establish an scientific and effective drug supervision and administration system.
Stimulate the enthusiasm of drug research and development
"" clear, the pilot within the administrative area of drug research and development institutions or scientific researchers can as a drug registration applicant submit drug clinical trial application, drug listing application, the applicant to obtain drug marketing license and drug approval number can become a drug marketing license holders.
"Under this mechanism, the listing permit holders and the production permit holders may be the same subject, or two independent entities." Wang Chenguang analysis said that, according to their own situation, the listing permit holders can produce their own, but also can be entrusted to other production enterprises. If the principal production, the marketing authorization holder in accordance with the law on drug safety, effective and quality controllable of negative full responsibility, production enterprise according to entrust the production contract provisions on drug quality of the marketing authorization holder is responsible for. This is also the general practice of drug management system in the international community.
In fact, listed license holder system and current drug registration system is the biggest difference lies not only in approval of the medicines main body of the paper by the pharmaceutical production enterprises expand to the medicine research and development institutions, scientific research personnel, and responsible for drug quality from beginning to end, the subject is also more clearly, which is conducive to the improvement of drug quality, improve the enthusiasm of the drug development and innovation.
It is understood, drug from R & D to obtain approval number needs huge investment and long cycle, the product approval for production line has been idle. If there is a problem in the approval process, then the production line will be put into a waste.
Wang Chenguang that drugs listed separation permits and production management mode, on the one hand a help in research and development, and focus on capital, technology and human resources for continued research and new drug research and development; on the other hand, help to clear and strengthen the research and development person in drug research and development, production, and use of the whole cycle bear the corresponding legal responsibility, prompting the continuous improvement and perfection of technology, ensuring the safety of drugs. More important is that research and development will help to become a marketing authorisation holder through technology transfer, contract manufacturing or other forms of drug production, improve equipment utilization, promoting specialization, research closely, change the passive situation of insufficient of our drug development input.
Clear stakeholder responsibility
The pilot of the drug listing license holder system marks the deepening of the reform of China's drug registration system, especially the obligations and responsibilities of the stakeholders in the system of drug listing license holders.
"Plan" proposed holder is the responsibility subject of the drug marketing license, to bear the full life cycle of a drug efficacy and safety guarantee obligations, including registration, production, circulation, monitoring and evaluation, the quality traceability, information disclosure, some obligations can and production enterprise agreed, but ultimate responsibility by the holders of the bear.
In this regard, the State Food and drug administration, Senior Research Institute expert Yang Yue believes that the pilot program to strengthen the main responsibility of the applicant and the holder, the establishment of the interests of drug quality and safety responsibility for the investigation of the chain. This also contributed to the applicant in the choice of R & D, production, sales and other partners will be more careful, to avoid future drug quality and safety law liability disputes, "can be expected, the pilot program will help to the establishment of the enterprise and individual behavior norms and the integrity of the system." Yang Yue said.
For the vast number of consumers, drug market license holders system can better protect the legitimate rights and interests of drug users. In short, when approved drugs caused personal injury, holders, commissioned the production of enterprises, the seller will bear legal liability, consumers can to which any claim for compensation; compensation, compensation from the party again according to the actual attribution of responsibility according to law to bear the responsibility of the party recover.
"This system will be designed to protect the legitimate rights and interests of consumers, regardless of the problem is the drug life cycle in which the consumer interests will be the first to be reasonably maintained." Wang Chenguang, said from the general experience of the drug R & D and production, marketing approval holder is the main R & D, the most thorough understanding of drug efficacy and quality, it has the regulatory capacity of the production, operation and use of, so will its positioning as primary liability for quality in full compliance with the rules of drug risk control.
Read news hot spots, showing sensitive events, more exclusive analysis, as in the "things" WeChat, scan two-dimensional code free reading.
|


